Introduction
Social stress is a type of hierarchical stress in which an individual has little or no ability to change his/her circumstances. It is considered a crucial factor in the transition between recreational drug use and addictive drug abuse. My scholarly project focuses on understanding the neural biochemical signaling pathways involved in the relationship between intermittent social stress, drug addiction, and behavior modification. In rats, it has been shown that intermittent social stress augments the effects of psychomotor stimulants such as amphetamine, a process called “cross-sensitization.”
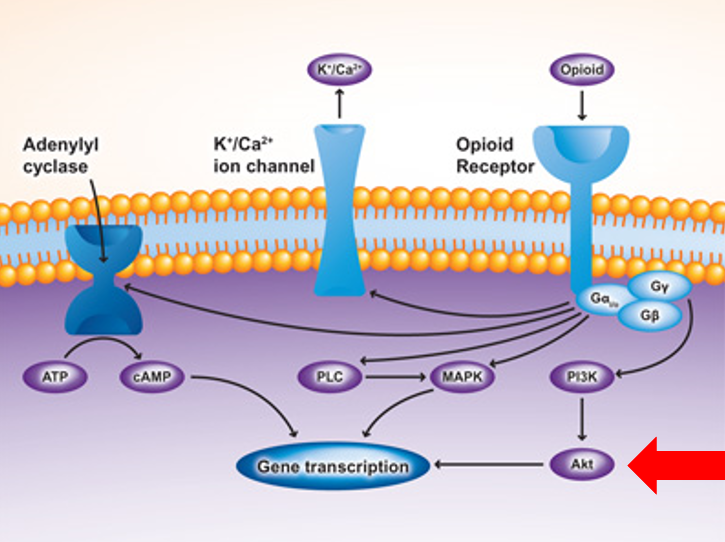
Cross-sensitization takes place in the ventral tegmental area (VTA) of the brain, a major component of the reward-pleasure pathway. After being exposed to social stress, cross-sensitization occurs predominantly due to the upregulation of mu-opioid receptors (MORs) in the VTA. Upregulation of MORs in the VTA is partially mediated through the activity of pAKT, a serine-threonine kinase (Figure 1). Previous studies have shown that pharmacologic inhibition of pAKT reduced cross-sensitization in rats that were exposed to intermittent social stress and then administered amphetamine (which binds to MORs).
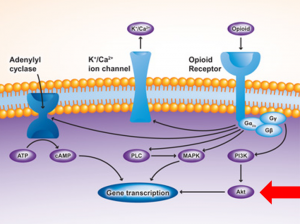
Figure 1: Some of the major biochemical pathways involved in changes in gene transcription (e.g., upregulation of MORs) after binding of amphetamine.
This pharmacologic inhibitor, however, was not specific to the VTA or pAKT. In my project, we use a herpes simplex virus that is embedded with a gene that leads to the transcription of a protein that specifically inhibits pAKT. In addition, we inject this virus directly into the VTA using stereotaxic surgery. Our hypothesis is that by injecting this so-called “dominant negative” pAKT virus into the VTA, we can prevent cross-sensitization from happening in intermittently socially stressed rats that have been given amphetamine.
Study design
NOTE: This experiment was performed twice, in Summer 2017 and in Fall 2017; data in the “Results” section are pooled from these two experiments.
There were 4 groups of 5 rats, for a total of 20 rats. Two groups of rats were intermittently socially stressed 4 times over a period of 10 days. The other two groups of rats were not intermittently socially stressed during that same period and were just “handled.” At the end of the 10 days, all rats underwent stereotaxic surgery. Of the 2 groups of stressed rats, one group received the virus and the other group did not. Of the 2 groups of “handled” rats, one group received the virus and the other group did not. After three days of post-operative monitoring, all the rats were enrolled in the “amphetamine challenge.” Low-dose intraperitoneal injections of amphetamine were given to each rat after an acclimation period with saline injection. Their locomotor activity was recorded by an overhead camera that measured and tracked heat signatures. After the amphetamine challenge, the rats were euthanized, and their brains were examined so that the injection tracks could be visualized (Figure 2).
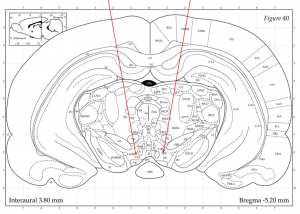
Figure 2: Schematic representation of stereotaxic surgery. The red lines indicate the ideal track lines into the brain, targeting the VTA.
Results
Data from the amphetamine challenge were analyzed (Figure 3). Stressed rats not given the virus (green line) demonstrated cross-sensitization to amphetamine (i.e., had an enhanced response to it). Stressed rats given the virus (black line) did not demonstrate cross-sensitization (had a blunted response to amphetamine).
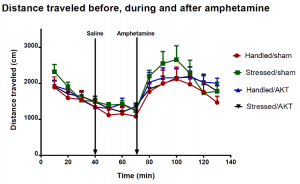
Figure 3: Graph of the results of the repeated amphetamine challenge for all 4 groups of rats. “Sham” refers to a surgery in which no virus was infused, but needle tracks were made. “AKT” refers to viral infusion. Error bars are shown. “Saline” and “Amphetamine” lines represent time points where injections were made. The total number of subjects in each group was 8, except “Handled/AKT,” where n = 5 (all from the repeated experiment).
Discussion
Despite a relatively small number of data points, it would seem logical to conclude that viral knockdown of pAKT in the VTA did attenuate cross-sensitization to amphetamine after intermittent social stress. However, it should be noted that the data were not statistically significant at any time point. Therefore, these results represent preliminary data that will require repeated experiments in order to increase the sizes of the subject groups and determine statistical significance.
- Yap JJ, Chartoff EH, Holly EN, Potter DN, Carlezon WA, Miczek KA. Social defeat stress-induced sensitization and escalated cocaine self-administration: The role of ERK signaling in the rat ventral tegmental area. Psychopharmacology (Berl ). 2015;232(9):1555-1569. doi: 10.1007/s00213-014-3796-7.
- Johnston CE, Herschel DJ, Lasek AW, Hammer RP, Nikulina EM. Knockdown of ventral tegmental area mu-opioid receptors in rats prevents effects of social defeat stress: Implications for amphetamine cross-sensitization, social avoidance, weight regulation and expression of brain-derived neurotrophic factor. Neuropharmacology. 2015;89:325-334. doi: 10.1016/j.neuropharm.2014.10.010.
- Wang J, Bastle RM, Bass CE, Hammer RP, Neisewander JL, Nikulina EM. Overexpression of BDNF in the ventral tegmental area enhances binge cocaine self-administration in rats exposed to repeated social defeat. Neuropharmacology. 2016;109:121-130. doi: 10.1016/j.neuropharm.2016.04.045.
- Johnston, C, Herchel, D, Lasek, A, Hammer, R, Nikulina, E. Phosphorylation of AKT in VTA GABA neurons: Implications for social defeat stress-induced vulnerabilities to amphetamine and deficits in weight gain in rats. Department of Neuroscience, Scripps Research Institute, Ph.D. thesis. 2015.
- Beloate LN, Omrani A, Adan RA, Webb IC, Coolen LM. Ventral tegmental area dopamine cell activation during male rat sexual behavior regulates neuroplasticity and d-amphetamine cross-sensitization following sex abstinence. J Neurosci. 2016;36(38):9949-9961. doi: 10.1523/JNEUROSCI.0937-16.2016.
Zachary Morrison is a medical student at the UA COM-Phoenix. He was born in Massachusetts and lived there for 13 years before moving to Arizona. He loves being in Arizona, and one of his favorite hobbies is rock climbing. He received a BS in medicinal biochemistry from ASU and worked as a scribe in the emergency department at Banner Baywood Hospital during his gap year. His main career interests now include emergency medicine, cardiology, and orthopedic surgery. Additionally, he has always been interested in how drugs affect the brain. Since he also likes procedural things, he decided to do his scholarly project on a topic that meshed the two together.