 CRISPR/Cas9 was recently named the scientific breakthrough of 2015 by the journal Science [1]. Said to be a revolutionary gene-editing technique, CRISPR/Cas9 has risen to fame on national media over the last several months. But the reality is that CRISPR (a pattern of short, repeating, palindromic DNA sequences) was first discovered in 1987 by a group of Japanese scientists in search of a protein-coding gene in E. coli [2]. It was not for another decade that scientists realized these repeats were present in many bacteria, and in 2012, the term CRISPR (clustered regularly interspaced short palindromic repeats) was coined [3]. Soon thereafter, scientists found CRISPR, along with CRISPR-associated (Cas) proteins, and RNA molecules play a significant role in protecting bacteria from viral infections. Named the CRISPR/Cas system, the discovery is now being studied intensely in laboratories across the world for its untapped yet promising revolutionary potential.
CRISPR/Cas9 was recently named the scientific breakthrough of 2015 by the journal Science [1]. Said to be a revolutionary gene-editing technique, CRISPR/Cas9 has risen to fame on national media over the last several months. But the reality is that CRISPR (a pattern of short, repeating, palindromic DNA sequences) was first discovered in 1987 by a group of Japanese scientists in search of a protein-coding gene in E. coli [2]. It was not for another decade that scientists realized these repeats were present in many bacteria, and in 2012, the term CRISPR (clustered regularly interspaced short palindromic repeats) was coined [3]. Soon thereafter, scientists found CRISPR, along with CRISPR-associated (Cas) proteins, and RNA molecules play a significant role in protecting bacteria from viral infections. Named the CRISPR/Cas system, the discovery is now being studied intensely in laboratories across the world for its untapped yet promising revolutionary potential.
The novelty of CRISPR is that it can change germline DNA whereas previous gene-editing tools have only been able to edit the genome of an individual. In other words, CRISPR gives us the power to fix abnormal DNA sequences before they are passed down to future generations. For example, Friedreich ataxia causes progressive damage of the nervous system leading to ataxia, diabetes, and scoliosis. Using CRISPR, we can locate and fix the GAA repeats associated with Friedreich ataxia in both parents’ genomes before conception. Furthermore, CRISPR’s ease of use makes it much more powerful and faster to use than its predecessors, making it possible for any size laboratory to experiment with the gene-editing tool.
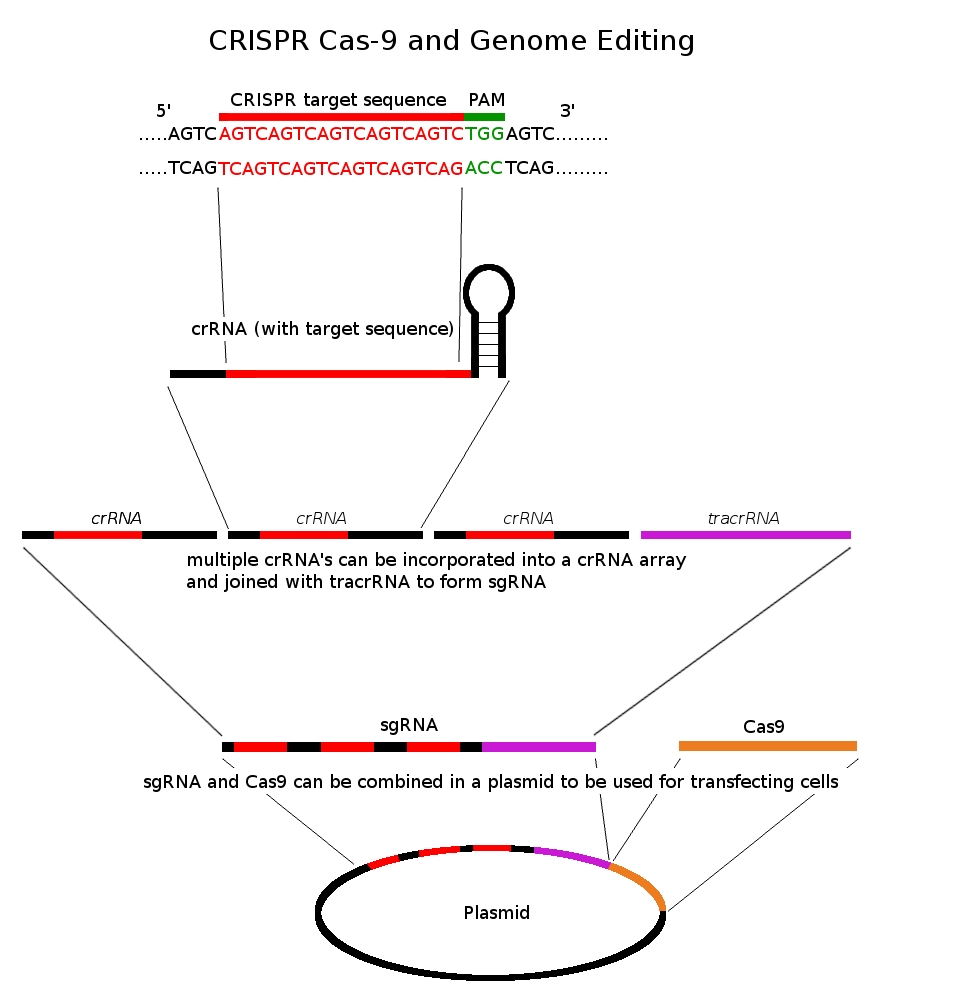
How does CRISPR work? When a virus attacks a bacterium, segments of the viral DNA are inserted into the bacterial genome as “place holders” between the short palindromic repeats of CRISPR. The newly inserted segments of viral DNA provide a template for RNA molecules to quickly identify and target the same segments of viral DNA in future viral infections. Once an incoming viral attack is detected, RNA molecules guide the CRISPR/Cas system to the viral sequence, where Cas proteins cleave the viral DNA and disable further viral propagation.
The reason why the CRISPR/Cas system has garnered so much attention recently is because of a study published in 2012 in which researchers were able to harness the power of the CRISPR/Cas system to make a new gene-editing tool [4]. The new gene-editing system, now called CRISPR/Cas9, uses a special type of RNA molecule that can be programmed to identify, disable, and or replace any gene sequence the programmer desires. With this novel implementation of CRISPR, the world of genetic engineering seems limitless. Anything from congenital and inherited diseases to genetically modified crops and cancer treatment. Rapid eradication of viral epidemics and potentially the elimination of many diseases from the human species. Think about the possibilities: Huntington disease, sickle cell anemia, thalassemia, Duchenne muscular dystrophy; the list goes on and on [5]. So is anything fair game?
With the power to change the human genome in such profound ways, there are a myriad of ethical, and moral, issues that should be discussed. It is likely only a matter of time before people will want the CRISPR/Cas9 system to design babies, boost intelligence, and create future athletes with super strengths. Although the possibilities seem endless, as of yet, we are just beginning to realize the true power of the CRISPR/Cas9 system.
- http://www.sciencemag.org/topic/2015-breakthrough-year
- Ishino Y, Shinagawa H, Makino K, Amemura M, Nakata A. Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product. J Bacteriol. 1987;169(12):5429-33.
- Jansen R, Embden JD, Gaastra W, Schouls LM. Identification of genes that are associated with DNA repeats in prokaryotes. Mol Microbiol. 2002;43(6):1565-75.
- Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816-21.
- Yin, Steph. “What Is CRISPR/Cas9 and Why Is It Suddenly Everywhere?” Motherboard. N.p., 30 Apr. 2016. Web. 13 Feb. 2016.
Dor Shoshan is an MS1 at The University of Arizona College of Medicine – Phoenix. He graduated from Chapman University in 2015 with a Bachelor of Science in biological sciences. He is passionate about the intersection of science and technology with medicine—in particular, the way technology will shape the future of medicine and its perils. For recommendations about future articles, comments or questions, please do not hesitate to contact at shoshan[at]email.arizona.edu