Congenital heart disease is a fascinating field that incorporates embryology, physiology, pathology, pediatrics, medicine, and interventions. The healthy human heart develops in utero through a symphony of loops, septations, twists, and buttons as well as shunts and compactions. Sometimes this complex sequence of events goes awry, and 1% of children are born with a congenital heart defect ranging from innocent flaps that close on their own to emergent crises requiring immediate intervention [1-2]. About 1 out of every 5000 live births results in one of the more serious conditions known as hypoplastic left heart syndrome [3].
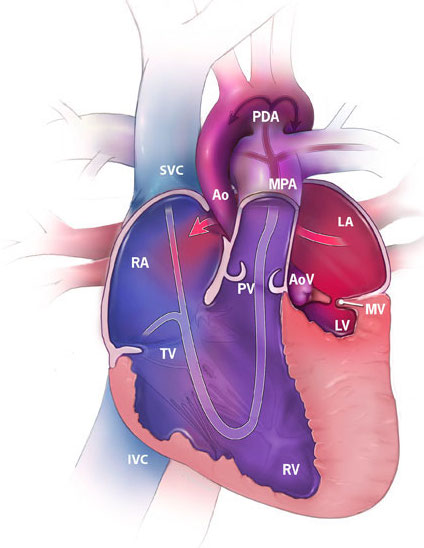
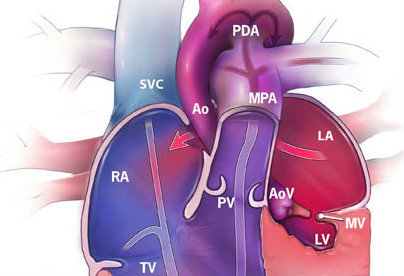
Figure 1. Hypoplastic left heart syndrome illustrating a hypoplastic left ventricle (LV), mitral valve (MV), aortic valve (AoV), and ascending aorta (Ao) (“Facts about…”, 2014).
Hypoplastic left heart syndrome (HLHS) is a congenital condition in which left heart structures, including the left ventricle, mitral valve, aortic valve, and ascending aorta are underdeveloped (Figure 1). Ultimately children born with HLHS have only one functional ventricle, and without intervention, HLHS is incompatible with life. Until 30 years ago, mortality rates were 100% for these children, and the only treatment was comfort care [4]. In 1983, Dr. Norwood introduced a surgical treatment for HLHS, and the survival rates for children born with HLHS are now as high as 76% [5]. This approach involves three separate open-heart operations over the first several years of life. The ultimate goals of these operations are to attach the systemic circulation to the only functional ventricle and to create a passive connection of venous blood flow that bypasses the heart and goes straight to the lungs.
While dramatically improving outcomes for these children, these three operations still carry significant morbidity and mortality rates. Additionally, some children are not healthy enough to survive the initial operation, which may take place when the child is less than a week old. A less-invasive approach to the first operation for HLHS was introduced in 1992 and was refined in the mid 2000s [6-9]. This so-called “hybrid approach” combines a catheter-based stent placement along with a less-invasive surgical procedure, taking the place of the first open-heart operation. While the child still must undergo the second and third open-heart operations, utilizing the hybrid approach for the first operation may allow for improved outcomes with less morbidity. The hybrid approach has been adopted by many centers and has been used for high-risk patients at St. Joseph’s Hospital and Phoenix Children’s Hospital since 2007.
My mentor, Joseph Graziano, MD, and I are investigating the outcomes for the hybrid approach to first-stage treatment of HLHS and hope to discover what factors positively and negatively influence these outcomes. The hybrid approach is a fairly recent development, and there is much to learn regarding reasons for re-intervention. In our study, we hypothesize that various physiologic and anatomic factors play a role in the re-intervention rates for the hybrid approach.
This scholarly project was a natural fit both for my interests and my background. I am interested in discovering new methods and developing new technologies to treat diseases and improve patient outcomes, and in this study, we investigate a new, minimally invasive approach to a procedure that is challenging both for the surgeon as well as the patient. Additionally, I worked as a medical device engineer for six years, the last three of which were spent designing and testing devices for congenital heart disease—a perfect segue to my project.
- Congenital Heart Defects, Data & Statistics. CDC website. Available at <http://www.cdc.gov/ncbddd/heartdefects/data.html>. Accessed 8/22/15
- Facts about hypoplastic left heart syndrome. Centers for Disease Control and Prevention website. Available at <http://www.cdc.gov/ncbddd/heartdefects/hlhs.html>. Published June 17, 2014. Accessed 8/28, 2014.
- Parker SE, Mai CT, Canfield MA, et al. Updated national birth prevalence estimates for selected birth defects in the united states, 2004-2006. Birth Defects Res A Clin Mol Teratol. 2010;88(12):1008-1016
- Feinstein JA, Benson DW, Dubin AM, et al. Hypoplastic left heart syndrome: Current considerations and expectations. J Am Coll Cardiol. 2012;59(1 Suppl):S1-42
- Ohye RG, Sleeper LA, Mahony L, et al. Comparison of shunt types in the Norwood procedure for single-ventricle lesions. N Engl J Med. 2010;362(21):1980-1992
- Akintuerk H, Michel-Behnke I, Valeske K, et al. Stenting of the arterial duct and banding of the pulmonary arteries: Basis for combined norwood stage I and II repair in hypoplastic left heart. Circulation. 2002;105(9):1099-1103
- Galantowicz M, Cheatham JP, Phillips A, et al. Hybrid approach for hypoplastic left heart syndrome: Intermediate results after the learning curve. Ann Thorac Surg. 2008;85(6):2063-70; discussion 2070-1
- Gibbs JL, Rothman MT, Rees MR, Parsons JM, Blackburn ME, Ruiz CE. Stenting of the arterial duct: A new approach to palliation for pulmonary atresia. Br Heart J. 1992;67(3):240-245
- Gibbs JL, Wren C, Watterson KG, Hunter S, Hamilton JR. Stenting of the arterial duct combined with banding of the pulmonary arteries and atrial septectomy or septostomy: A new approach to palliation for the hypoplastic left heart syndrome. Br Heart J. 1993;69(6):551-555
Daniel Crawford studied chemical engineering at The University of Arizona in Tucson, AZ. After graduating, he worked for 6 years as a biomedical device engineer in Flagstaff, AZ for W.L. Gore & Associates, Inc. His work included manufacturing engineering, process development, device design, and device testing. He is currently an MS2 student at The University of Arizona College of Medicine – Phoenix.