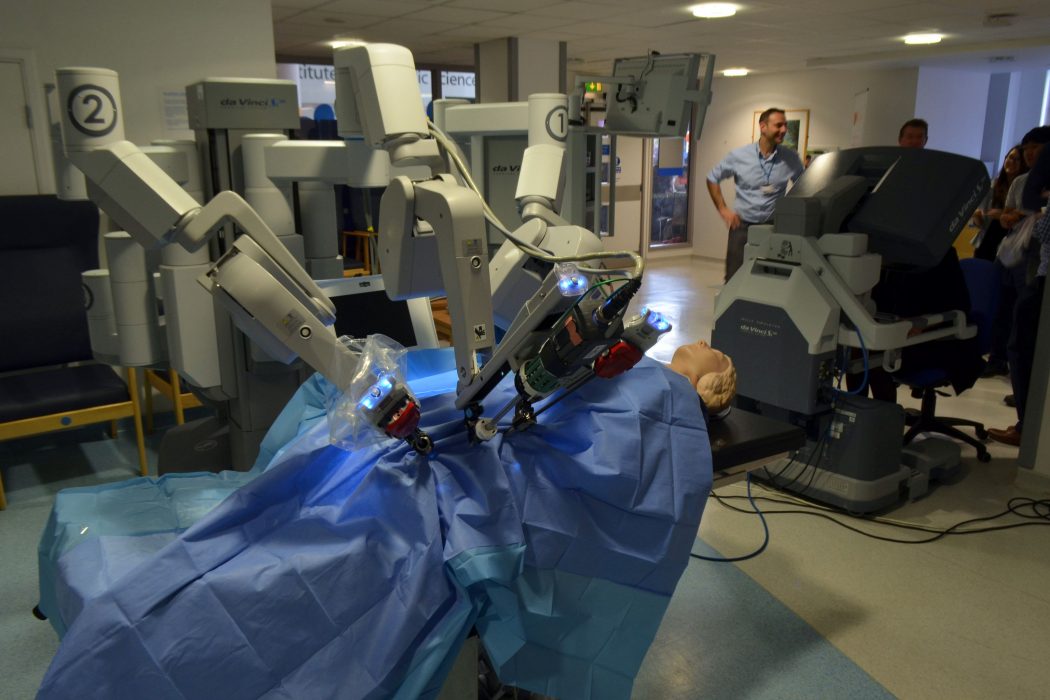
Fifteen years after its creation, the da Vinci Surgical System is as futuristic, innovative, and revolutionary as ever before. Designed by the United States Army and Stanford University, the initial idea was for an armored robot to roll into the battlefield where a surgeon could perform surgery out of harm’s way but still on the frontlines. After its inception, researchers quickly discovered it was not fit for the task – the system was too large, too slow and too costly for the fast-moving destructive frontlines of a battlefield. Although the system was not suited for the army, developers improved the technology and adapted it for civilian hospitals. In 2000, the U.S. Food and Drug Administration (FDA) approved the da Vinci Surgical System for use in various procedures, including urologic, gynecologic, cardiac, colorectal, and general laparoscopic surgeries.
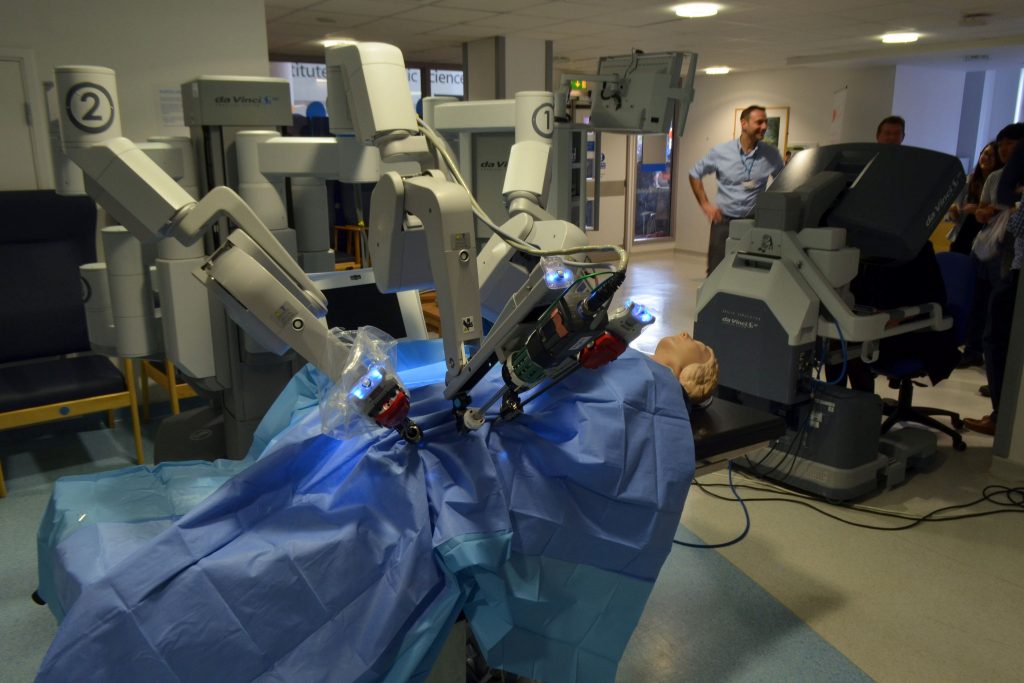 The benefits of using the da Vinci Surgical System are, on average: shorter hospital stay, less blood loss, fewer complications, less need for narcotic pain medication, faster recovery, and smaller incisions for minimal scarring [1-11]. Drawbacks, however, are plentiful. Robotic surgery is difficult to learn, and the 2 million U.S. dollars investment and maintenance costs present a significant burden for many hospitals, which renders this system out of reach for countless institutions.
The benefits of using the da Vinci Surgical System are, on average: shorter hospital stay, less blood loss, fewer complications, less need for narcotic pain medication, faster recovery, and smaller incisions for minimal scarring [1-11]. Drawbacks, however, are plentiful. Robotic surgery is difficult to learn, and the 2 million U.S. dollars investment and maintenance costs present a significant burden for many hospitals, which renders this system out of reach for countless institutions.
Since its approval, “physicians have used the da Vinci System successfully worldwide in approximately 1.5 million various surgical procedures to date” [12]. To buy one of these systems costs a little under 2 million U.S. dollars, excluding the hundreds of thousands of dollars necessary for yearly maintenance. The steep price, however, has not slowed demand. Currently, there are more than 2,153 units in the U.S., 499 in Europe, 183 in Japan, and 267 others spread throughout the world [13].
The system is made up of three consoles: the surgeon’s console, the robotic console, and an HD 3D vision system for proctoring [12]. When sitting at the console, the surgeon sees a remarkably clear 3D image of the surgical field, mimicking the stereoscopic vision of normal human eyesight. Surgeons insert their fingers into gloves that scale, filter, and translate movements to the robotic console. The system is smart enough to filter hand tremors or shaking and precise enough to reduce several centimeters of movement to several micrometers on the robotic terminal. Furthermore, the robotic console relays tactile feedback to the surgeon so that he or she can feel any tenderness, stiffness, or even the tension of a suture, and these parameters can be quantified on the surgical display. These and many more features lead to an incredibly immersive surgical experience that is unmatched by traditional bedside surgery.
Fifteen years after its clinical approval and first implementation, the da Vinci Surgical System is still the shiny, new toy that hospitals tout, and rightfully so.
- Park JS, et al. S052: a comparison of robot-assisted, laparoscopic, and open surgery in the treatment of rectal cancer. Surg Endosc. 2011 Jan;25(1):240-8. Epub 2010 Jun 15.
- Poston RS, et al. Comparison of economic and patient outcomes with minimally invasive versus traditional off-pump coronary artery bypass grafting techniques. Ann Surg. 2008 Oct;248(4):638-46.
- Health Information and Quality Authority (HIQA), reporting to the Minister of Health-Ireland. Health technology assessment of robot-assisted surgery in selected surgical procedures, 21 September 2011.
- Landeen LB, et al. Clinical and cost comparisons for hysterectomy via abdominal, standard laparoscopic, vaginal and robot-assisted approaches. S D Med. 2011 Jun;64(6):197-9, 201, 203 passim.
- de Souza AL, et al. A comparison of open and robotic total mesorectal excision for rectal adenocarcinoma. Dis Colon Rectum. 2011 Mar;54(3):275-82.
- Cerfolio RJ, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg. 2011 Oct;142(4):740-6. Epub 2011 Aug 15.
- Shaligram A, et al. How does the robot affect outcomes? A retrospective review of open, laparoscopic, and robotic Heller myotomy for achalasia. Surg Endosc. 2012 Apr;26(4):1047-50. doi: 10.1007/s00464-011-1994-5. Epub 2011 Oct 25.
- Lowe MP, et al. A comparison of robot-assisted and traditional radical hysterectomy for early-stage cervical cancer. Journal of Robotic Surgery 2009:1-5.
- Menon M, et al. Prospective comparison of radical retropubic prostatectomy and robot- assisted anatomic prostatectomy: the Vattikuti Urology Institute experience. Urology. 2002 Nov;60(5):864-8.
- Bell MC, et al. Comparison of outcomes and cost for endometrial cancer staging via traditional laparotomy, standard laparoscopy, and robotic techniques. Gynecologic Oncology III 2008:407-411.
- Miller J, et al. Prospective evaluation of short-term impact and recovery of health related quality of life in men undergoing robotic assisted laparoscopic radical prostatectomy versus open radical prostatectomy. J Urol. 2007 Sep;178(3 Pt 1):854-8; discussion 859. Epub 2007 Jul 16.
- http://www.davincisurgery.com
- http://phx.corporateir.net/phoenix.zhtml?c=122359&p=irol-faq
Dor Shoshan is an MS1 at The University of Arizona College of Medicine – Phoenix. He graduated from Chapman University in 2015 with a Bachelor of Science in biological sciences. He is passionate about the intersection of science and technology with medicine—in particular, the way technology will shape the future of medicine and its perils. For recommendations about future articles, comments or questions, please do not hesitate to contact at shoshan[at]email.arizona.edu